More than 60 forums and conferences
More than 800 industry leaders, industry elites and opinion leaders
Nearly 200,000 square meters of exhibition and conference area
Nearly 4,000 brand companies, with tens of thousands of products on display
More than 120,000 professional visitors to visit
Gathering the world's cutting-edge "high-tech" blockbuster products
Fully display the most cutting-edge trends in the global medical device industry
Leading the industry's new future and new direction
......
That's right, the global medical industry's "aircraft carrier" - the 90th China International Medical Equipment Fair (CMEF), has come!

After 45 years of development, CMEF has grown into a global comprehensive service platform that covers the entire industry chain and the entire life cycle, integrating product technology, new product launches, trade cooperation, and academic exchanges. It is known as the "weather vane" of the global medical industry! The 90th CMEF closely follows the pulse of the industry, focusing on new scenarios and new markets for tens of thousands of products with precise vision, expanding new channels of the industrial ecology with a broader vision, and digging for gold in overseas markets with a more forward-looking perspective to share new opportunities!



As one of the star companies in the Daxing Biopharmaceutical Industry Base, Biosis Healing came to the Shenzhen CMEF event with the CBP China Pharmaceutical Valley Enterprise Delegation, and brought its blockbuster biosis products- Boreiqiang® Vidasis SIS Stapleline Reinforcement, Boreijin® Biological Absorbable Dental Membrane, and Boreimei® Dural repair patch to the conference, accepting the "review" of medical practitioners from all over the world.
Exhibition time: October 12-15, 2024
Exhibition location: Shenzhen International Convention and Exhibition Center (Bao'an)
CBP China Pharmaceutical Valley Booth No.: Hall 12 S26






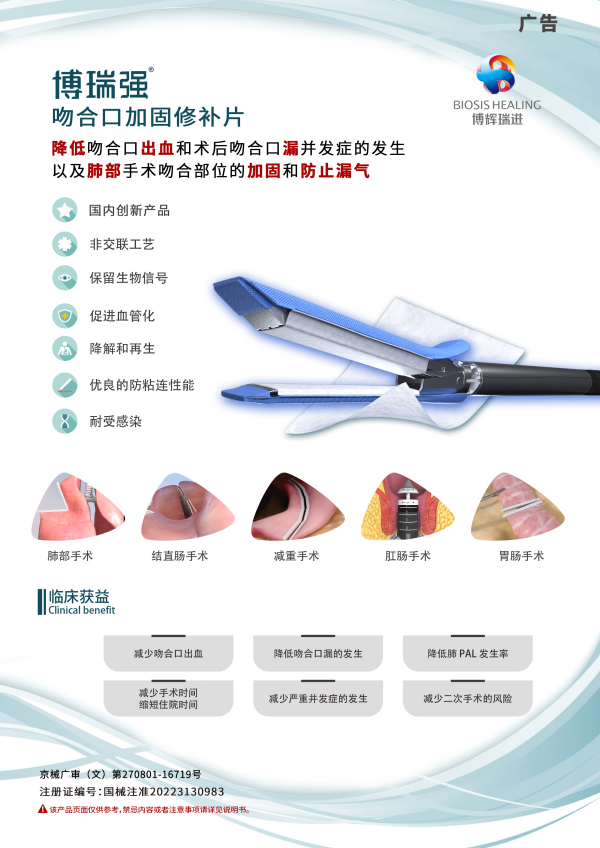
Borreqiang® Vidasis SIS Stapleline Reinforcement is a product of the special approval channel for innovative medical devices of the State Food and Drug Administration (No. 1, 2018), made of SIS materials with independent intellectual property rights of Biosis Healing. The product can be used with all mainstream brands of staplers on the market to reinforce the anastomotic site, effectively reducing the occurrence of anastomotic bleeding and postoperative anastomotic leakage complications in gastrointestinal surgery, as well as the reinforcement of the anastomotic site and preventing air leakage in lung surgery.
The material is rich in bioactive substances, accelerates the healing of the anastomosis, has no cross-linking agent, and the implanted material can be completely degraded and absorbed after tissue regeneration. Fewer electrocoagulation hemostasis operations can save doctors time for intraoperative hemostasis and suture reinforcement. Excellent material properties and suture retention, no contracture after implantation, no stenosis of the anastomosis site, and reduced damage caused by suture cutting tissue. Light, thin and uniform material thickness does not affect the stapler firing operation and staple forming.
The use of Vidasis SIS Stapleline Reinforcement during surgery can achieve hemostasis and anastomotic reinforcement through physical reinforcement and endogenous induced tissue repair, reduce the incidence of postoperative bleeding and anastomotic leakage, shorten the operation and hospitalization time, and accelerate patient recovery.
Borregen® Biological Absorbable Dental Membrane
Borregen® Biological Absorbable Dental Membrane is made of non-cross-linked porcine small intestinal submucosal extracellular matrix material (hereinafter referred to as "SIS material") with independent intellectual property rights of Biosis Healing. SIS material has the advantages of good biocompatibility, promoting tissue regeneration and repair, accelerating angiogenesis, and tolerating infection. It is prepared by non-cross-linking process, without the addition of chemical reagents, and the product can be completely degraded and absorbed with tissue regeneration, without foreign matter residue. It is widely used in the repair and reconstruction of tendons, dura mater (spinal) membrane, abdominal wall, skin, oral cavity and other tissues.
Borregen® Biological Absorbable Dental Membrane can be used in combination with Bone Graft materials, and is suitable for physical barrier when oral tooth loss requires implantation and repair. The product has good biocompatibility and can be completely degraded with tissue regeneration, without affecting new bone formation and new bone quality; the double-sided structure design, the dense surface fibers are tight, which can prevent epithelial cells and connective tissue from growing into the bone defect area, and the barrier cycle can reach 8 weeks. The loose surface fibers are loose and have large pores, which are conducive to cell attachment. After implantation in the body, it can induce angiogenesis, guide bone regeneration, and form woven bone in the defect area within 6 weeks.
Borregen® Biological Absorbable Dental Membrane has good soft fit and hydrophilicity, which can better fit the defect area and facilitate surgical operation; it is rich in bioactive substances, which can promote cell attachment and tissue regeneration; it has excellent tolerance to infection and can reduce the chance of wound infection. It is a relatively ideal oral biomembrane product in clinical practice.

Borregen® Dural repair patch
Borregen® Dural repair patch is mainly used for the repair of dura (spinal) membrane defects. According to clinical data, for neurosurgery of the brain and spine, most patients need to use artificial patches to repair the defects of dura mater tissue, maintain the anatomical integrity of the central nervous system, protect the nerve center, prevent cerebrospinal fluid leakage and prevent adhesion.
Borime® Dural repair patch uses decellularized porcine small intestinal submucosal matrix material as raw material and is produced by non-cross-linking process to form a three-dimensional loose porous structure and retain natural bioactive ingredients. The product has similar characteristics to dura mater tissue, can effectively prevent cerebrospinal fluid leakage, has good biocompatibility, low inflammatory response, prevents tissue adhesion and tolerates infection. As the dura mater tissue regenerates, the product gradually degrades to complete absorption without chemical residues. The product has high mechanical strength, can be sewn or pasted, and is convenient for clinical surgery.