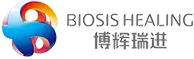Recently, Vidasis® Bone Graft Material products have been officially approved by the National Medical Products Administration for Class III medical device registration certificate [Registration Certificate Number: National Medical Device Registration No. 20243172284], adding another "capable member" to the Biosis Healing oral family!
Vidasis® Bone Graft Material is a composite of collagen and hydroxyapatite derived from the non-cross-linked porcine small intestinal submucosal extracellular matrix material (SIS material) with completely independent intellectual property rights of Biosis Healing. It is a Bone Graft Material with bioactive collagen. This product needs to be used in conjunction with a barrier membrane and is suitable for bone defect repair in dental surgery, including: filling of extraction sockets after tooth extraction and residual root extraction; restoration of alveolar ridges; and repair of alveolar bone defects caused by periodontal disease.
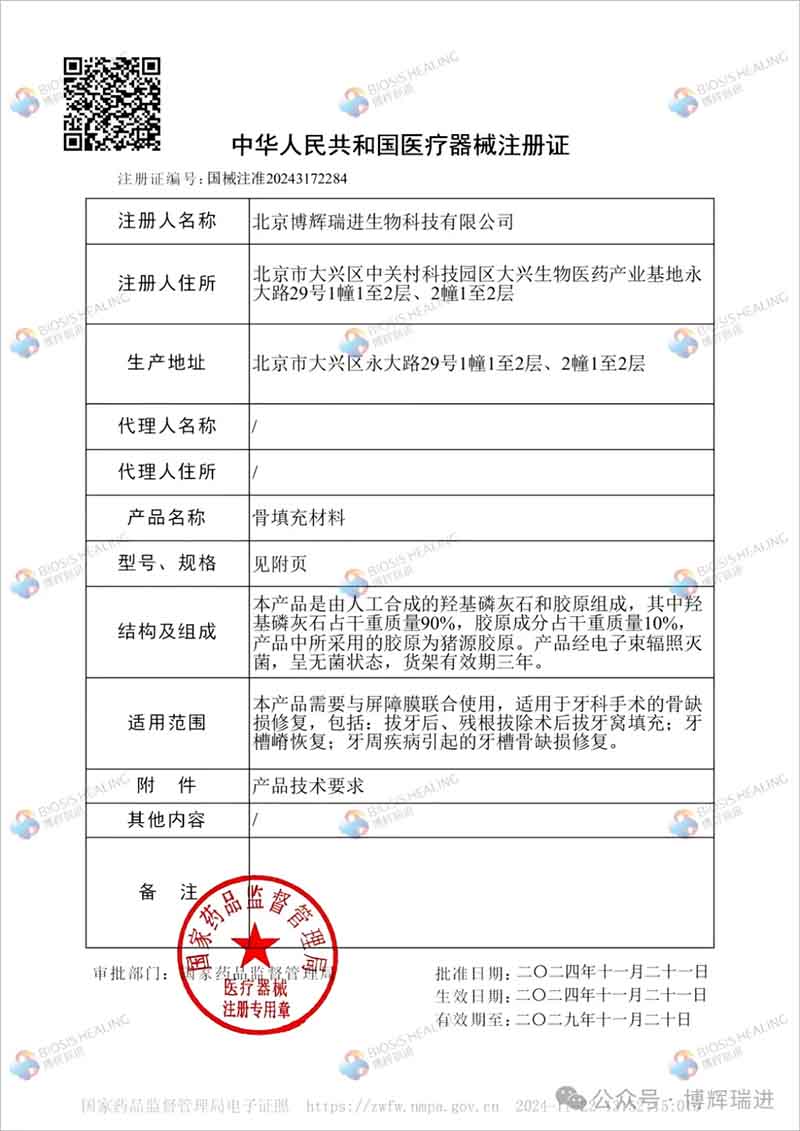
Vidasis® Bone Graft Material is rich in SIS bioactive collagen, and has the advantages of good biocompatibility, promoting tissue regeneration and repair, and accelerating angiogenesis. It can effectively promote bone repair and regeneration, promote cell growth, rapid vascularization, facilitate bone integration, reduce patient pain, and accelerate the healing process.
The unique elastic scaffold structure of Vidasis® Bone Graft Material can avoid deformation and displacement caused by external forces, and can increase the stability of the implanted material. The product advantages of good hydrophilicity and easy shaping make it easy for doctors to cut it at will according to their needs and match various forms of bone defects, making clinical operations more convenient and quicker.

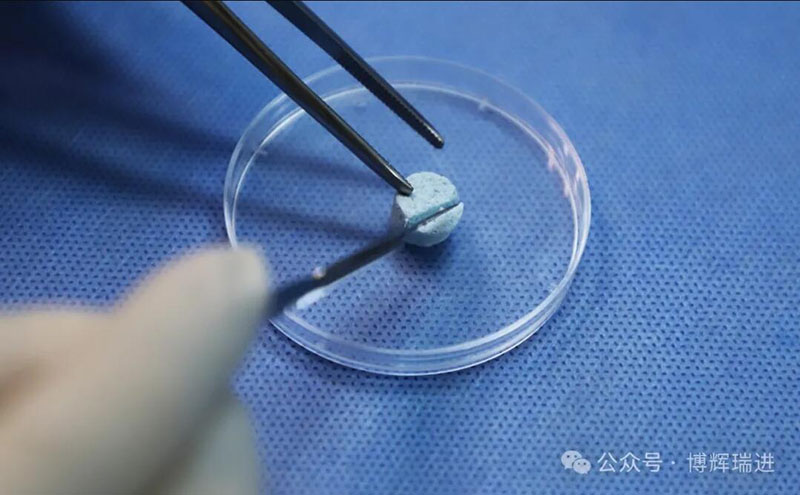
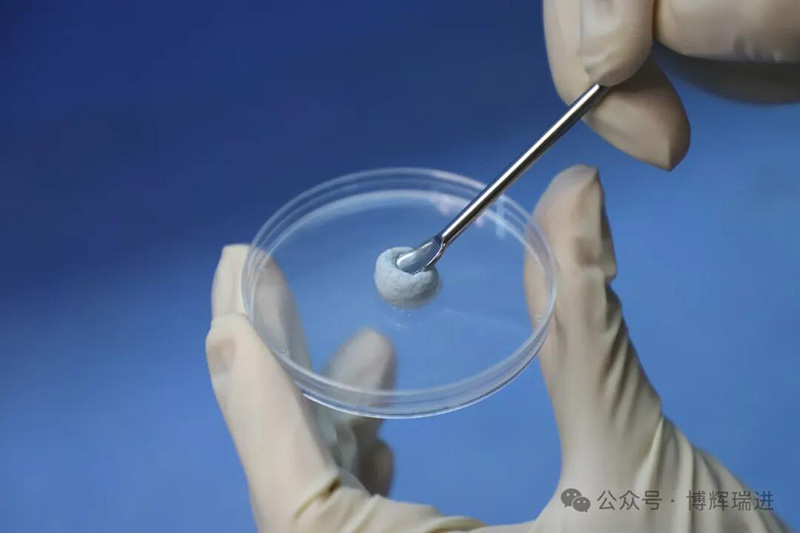
It has been more than two years since the certification of Vidasis® Oral Restoration Membrane in April 2022 and Biosis Healing officially entered the field of oral restoration. In the past two years, Biosis Healing oral products have won the recognition and trust of many doctors and patients with their excellent product performance, excellent clinical performance and absolute cost-effectiveness, and gradually established brand awareness and reputation in the oral field.
For a long time, the R&D team of Biosis Healing has always maintained close communication with oral clinical experts, listened carefully to the application feedback from the clinical front line, made fine adjustments to the biosis products, clarified the R&D direction of new products, and strived to provide clinical with a more effective and convenient product experience.
The approved Vidasis® Bone Graft Material has undergone rigorous clinical trials and quality testing to ensure safety and effectiveness in clinical use. The certification of this new product will further improve the product layout of Biosis Healing in the field of oral restoration. We have reason to believe that the best combination of Boragen® "Dental Membrane + Bone Graft Material", including Boragen® absorbable biological membrane, Oral Matrix + Bone Graft Material, will surely provide dentists with better, more comprehensive and more trustworthy solutions, bring patients more ideal treatment effects and more cost-effective treatment options, reshape patients' oral health, and open a new chapter in oral treatment.