Biological Hernia Graft
Biological Hernia Graft is an advanced product designed for hernia repair. It is a revolutionary solution that combines biological materials with state-of-the-art technology. This graft offers excepti...
Biological Hernia Graft
Biological Hernia Graft is an advanced product designed for hernia repair. It is a revolutionary solution that combines biological materials with state-of-the-art technology. This graft offers excepti...
 Dural Repair Patch
The dura mater (spinal) repair patch is made from the submucosal layer of porcine small intestine, forming a pale yellow sheet-like object that can degrade and be absorbed in the body.
Dural Repair Patch
The dura mater (spinal) repair patch is made from the submucosal layer of porcine small intestine, forming a pale yellow sheet-like object that can degrade and be absorbed in the body.
 Wound Matrix
Invention patent number: ZL 201310203594.1Non-crosslinking Decellularized Extracellular Matrix Derived Biomaterial: The SIS Wound Repair Matrix is an advanced wound management product,a natural scaff...
Wound Matrix
Invention patent number: ZL 201310203594.1Non-crosslinking Decellularized Extracellular Matrix Derived Biomaterial: The SIS Wound Repair Matrix is an advanced wound management product,a natural scaff...
 Biological Dental Membrane
Medical Device Registration Certificate Number: National Medical Device Registration Approval 20223170457.Prepared from non-crosslinked extracellular matrix-derived biomaterial (SIS).
Biological Dental Membrane
Medical Device Registration Certificate Number: National Medical Device Registration Approval 20223170457.Prepared from non-crosslinked extracellular matrix-derived biomaterial (SIS).
 SIS Staple Line Reinforcement (Gastric and Lung Resection)
SIS Staple Line Reinforcement (Gastric and Lung Resection)
 Disposable Endoscopic Linear Cutter Stapler
The disposable laparoscopic cutting stapler and its components consist of the main body and components, where the main body is composed of the firing rod assembly, inner barrel core assembly, outer ba...
Disposable Endoscopic Linear Cutter Stapler
The disposable laparoscopic cutting stapler and its components consist of the main body and components, where the main body is composed of the firing rod assembly, inner barrel core assembly, outer ba...
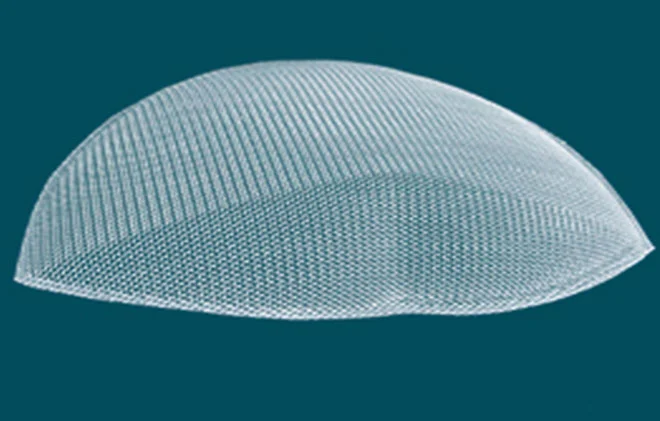 Laparoscopic 3D Polypropylene Mesh
Surgical mesh is a mesh fabric made of medical polypropylenemonofilament fibers, providing a comprehensive solution forhernia repair and soft tissue reconstruction.
Laparoscopic 3D Polypropylene Mesh
Surgical mesh is a mesh fabric made of medical polypropylenemonofilament fibers, providing a comprehensive solution forhernia repair and soft tissue reconstruction.
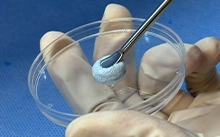 Bone Grafting Material
Vadisis Bone Graft is composed ofporcine collagen derivedfrom small intestine submucosa (SlS) and hydroxyapatite.1. SlS collagen material: used as a bioactive factor in scaffold materials to promote r...
Bone Grafting Material
Vadisis Bone Graft is composed ofporcine collagen derivedfrom small intestine submucosa (SlS) and hydroxyapatite.1. SlS collagen material: used as a bioactive factor in scaffold materials to promote r...
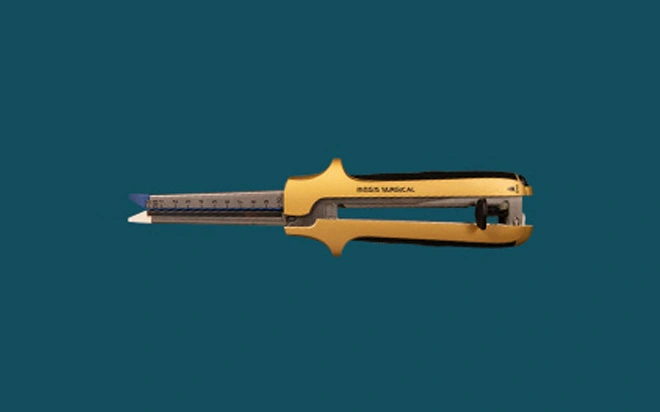 Disposable Linear Cutter Stapler
The disposable linear cutter stapler and its components consist of the stapler body and the cartridge assembly. The stapler body is composed of the firing handle assembly, the movable handle assembly,...
Disposable Linear Cutter Stapler
The disposable linear cutter stapler and its components consist of the stapler body and the cartridge assembly. The stapler body is composed of the firing handle assembly, the movable handle assembly,...
 SIS Staple Line Reinforcement (Gastric and Lung Resection)
Medical Device Registration Certificate Number: National Medical Device Registration Approval 20223130983 National Medical Products Administration Special Approval Pathway for Innovative Medical Devic...
SIS Staple Line Reinforcement (Gastric and Lung Resection)
Medical Device Registration Certificate Number: National Medical Device Registration Approval 20223130983 National Medical Products Administration Special Approval Pathway for Innovative Medical Devic...
 Disposable Circular Stapler
The medical device registration number is: Jingxie Residence Permit 20182020294Utility model: ZL201820551066.3Utility model: ZL201921063703.3The disposable tubular stapler mainly consists of a nail se...
Disposable Circular Stapler
The medical device registration number is: Jingxie Residence Permit 20182020294Utility model: ZL201820551066.3Utility model: ZL201921063703.3The disposable tubular stapler mainly consists of a nail se...
 Disposable Hemorrhoidal Stapler
The disposable anorectal stapler consists of an adjusting nut assembly, handle assembly, fixed handle casing, rear tightening ring, staple cartridge assembly (comprising a staple cartridge sleeve, sta...
Disposable Hemorrhoidal Stapler
The disposable anorectal stapler consists of an adjusting nut assembly, handle assembly, fixed handle casing, rear tightening ring, staple cartridge assembly (comprising a staple cartridge sleeve, sta...
 Disposable Hemorrhoids Stapler Plus
The disposable hemorrhoidal ligator consists of a hemorrhoid suction tube, a handle assembly (mainly composed of a fixed handle, a firing handle, a limit block, and a spring), a reset button, a suctio...
Disposable Hemorrhoids Stapler Plus
The disposable hemorrhoidal ligator consists of a hemorrhoid suction tube, a handle assembly (mainly composed of a fixed handle, a firing handle, a limit block, and a spring), a reset button, a suctio...
 Anal Fistula Plug
This product is made from the submucosal layer of porcine small intestine through a decellularization process, and is structured as a white or pale yellow conical object. The product is sterilized wit...
Anal Fistula Plug
This product is made from the submucosal layer of porcine small intestine through a decellularization process, and is structured as a white or pale yellow conical object. The product is sterilized wit...
 Disposable Circumcision Stapler
It consists of an anvil seat bracket (anvil seat, glans cover, spacer ring, bracket rod), an anastomosis cutting component (staple cartridge, staple cartridge protective cover, cutting knife, staple c...
Disposable Circumcision Stapler
It consists of an anvil seat bracket (anvil seat, glans cover, spacer ring, bracket rod), an anastomosis cutting component (staple cartridge, staple cartridge protective cover, cutting knife, staple c...